The Food and Drug Administration (FDA) issued recall notices for eye drop manufacturersPharmedicaandApotex, citing contamination concerns.
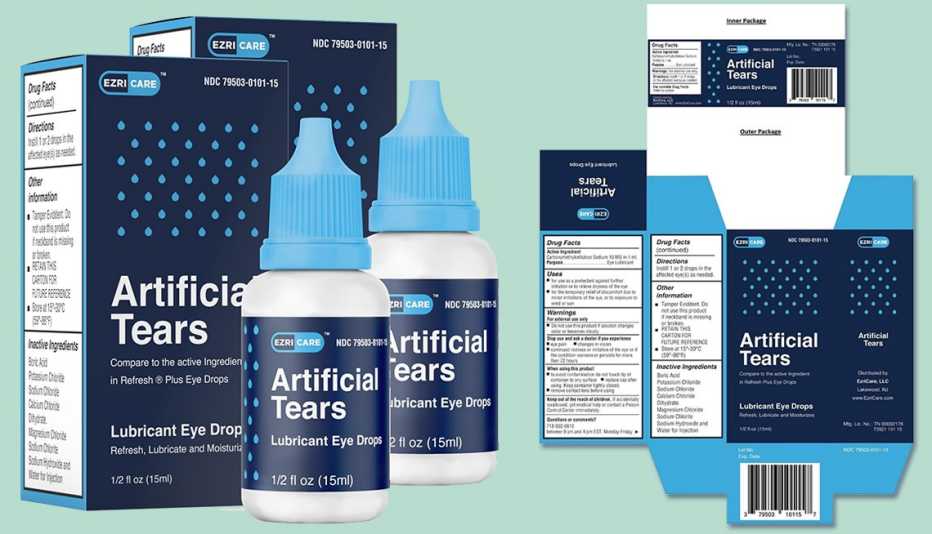
In February, EzriCare Artificial Tears wererecalledafter being linked to antibiotic-resistant infections.
At least 55 cases were reported, which resulted in vision loss, hospitalization and one death.
The products were sold worldwide at trade shows and at online retailers, including Amazon.com.
Pharmedica advises consumers to immediately stop using the recalled products and return them to the place of purchase.
The product is used by patients with open-angleglaucomaor ocular hypertension.
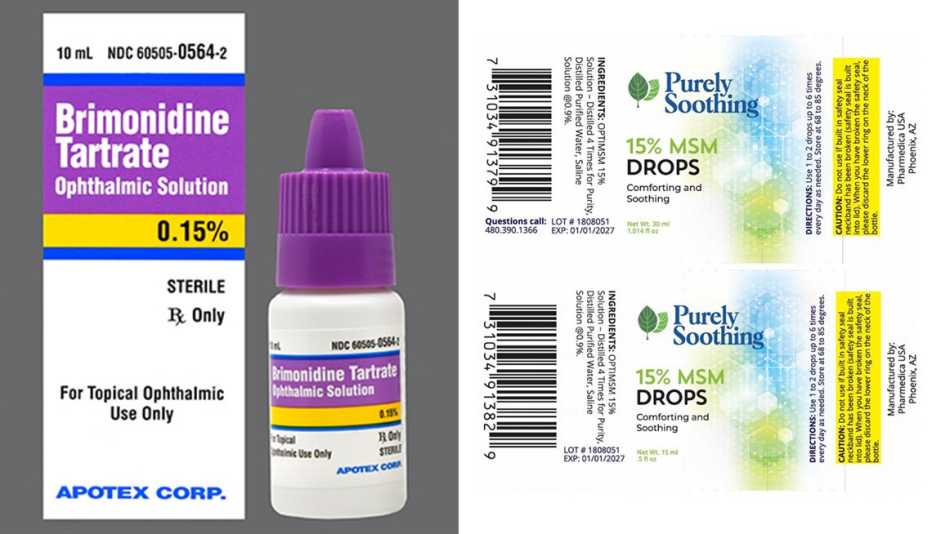
Patients who purchased the recalled lots should contact their pharmacy and their health care provider for medical advice.
The drops may be returned to Inmar Rx Solutions by calling 855-275-1273 from 9 a.m. to 5 p.m.
There have been no reports of injury or adverse events related to either of the newly recalled eye drops.

There are no known connections to the February recall of Ezricare Artificial Tears.